PHARMA CONSULTING
Advisory services from early stage to pre and post-market
We provide expertise in study protocol development, clinical trial application, and early stage labelling development for your investigational drug - helping you prioritize issues to guide your interactions with health authorities. We don't stop here. We help guide you through clinical studies and premarket approvals with global regulatory authorities.
FULL R&D SERVICES
Our full offerings guarantee product excellence
We understand how critical technical files are to the story of your products' development. This is why we dive deep under the hood to help technically write and structure your product master files to compliant formats, so that you satisfy initial reviews of your target health authority.
Product Lifecycle Management
We manage every stage of your products' lifecycle.

Quality Assurance
We provide critical inputs into developing quality management systems.

Current Good Manufacturing Practices
From technology transfers to site validation protocols, we are there through every step of the way.
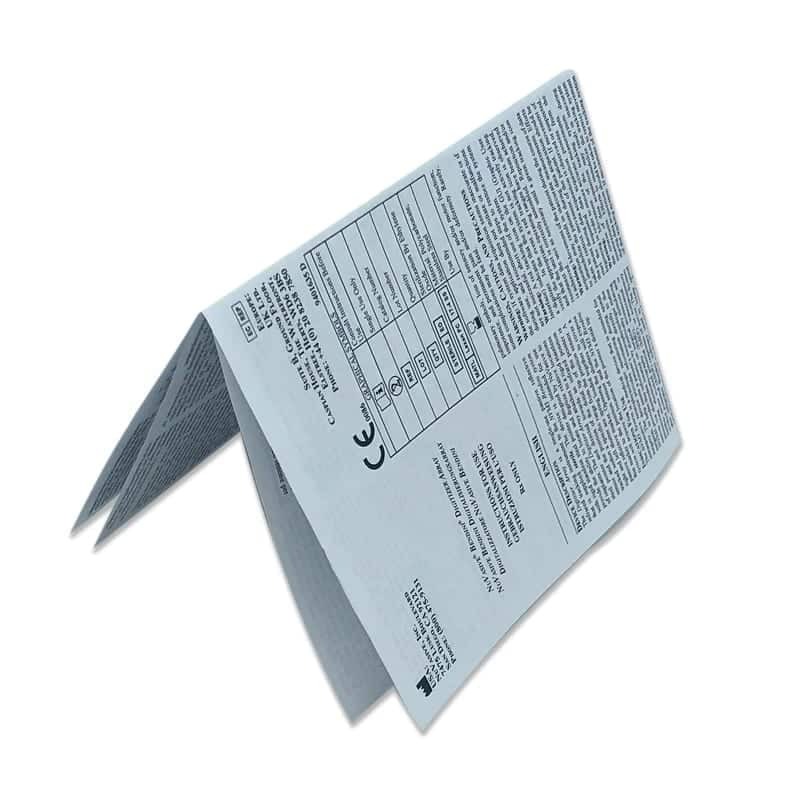
Labelling and Instructions For Use
From Target Label Profiles to Patient Information Leaflets, we are proficient at planning your product labelling process for all medicines and devices.
Some of our clients
We have been privileged to partner on many deeply rewarding and gratifying client projects over the past few decades. We do not take it for granted; this is why we continuously nurture these relationships and show how much we value them.